Page 128 - JSOM Fall 2025
P. 128
hemorrhage control device in a lethal swine model. Heliyon. 28. Schechtman DW, Kauvar DS, De Guzman R, et al. Differing resus-
2024;10(17):e37017. doi:10.1016/j.heliyon.2024.e37017 citation with aortic occlusion in a swine junctional hemorrhage
26. Zhang HY, Guo Y, Liu H, Tang H, Li Y, Zhang LY. Imaging anat- polytrauma model. J Surg Res. 2020;248:90–97. doi:10.1016/j.jss.
omy and surface localization of external control device- targeted 2019.11.028
arteries for noncompressible torso hemorrhage. Mil Med. 2022; 29. Schechtman DW, Kauvar DS, De Guzman R, et al. Abdominal
187(3–4):e343–e350. doi:10.1093/milmed/usab050 aortic and junctional tourniquet versus zone III resuscitative en-
27. Kuckelman JP, Barron M, Moe D, et al. Extending the golden dovascular balloon occlusion of the aorta in a swine junctional
hour for Zone 1 resuscitative endovascular balloon occlusion of hemorrhage model. J Trauma Acute Care Surg. 2020;88(2):292–
the aorta: improved survival and reperfusion injury with intermit- 297. doi:10.1097/TA.0000000000002553
tent versus continuous resuscitative endovascular balloon occlu-
sion of the aorta in a porcine severe truncal hemorrhage model. PMID: 40952909; DOI: 10.55460/ GHJ-K86Z
J Trauma Acute Care Surg. 2018;85(2):318–326. doi:10.1097/
TA.0000000000001964
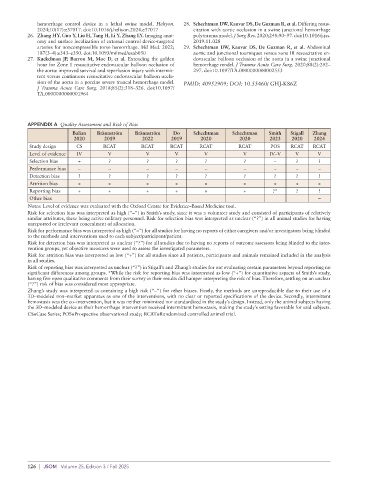
APPENDIX A Quality Assessment and Risk of Bias
Balian Brännström Brännström Do Schechtman Schechtman Smith Stigall Zhang
2020 2019 2022 2019 2020 2020 2023 2020 2024
Study design CS RCAT RCAT RCAT RCAT RCAT POS RCAT RCAT
Level of evidence IV V V V V V IV–V V V
Selection bias + ? ? ? ? ? – ? ?
Performance bias – – – – – – – – –
Detection bias ? ? ? ? ? ? ? ? ?
Attrition bias + + + + + + + + +
Reporting bias + + + + + + ?* ? ?
Other bias –
Notes: Level of evidence was evaluated with the Oxford Centre for Evidence–Based Medicine tool.
Risk for selection bias was interpreted as high (“–”) in Smith’s study, since it was a volunteer study and consisted of participants of relatively
similar attributes, these being active military personnel. Risk for selection bias was interpreted as unclear (“?”) in all animal studies for having
unreported or irrelevant concealment of allocation.
Risk for performance bias was interpreted as high (“–”) for all studies for having no reports of either caregivers and/or investigators being blinded
to the methods and interventions used to each subject/participant/patient.
Risk for detection bias was interpreted as unclear (“?”) for all studies due to having no reports of outcome assessors being blinded to the inter-
vention groups, yet objective measures were used to assess the investigated parameters.
Risk for attrition bias was interpreted as low (“+”) for all studies since all patients, participants and animals remained included in the analysis
in all studies.
Risk of reporting bias was interpreted as unclear (“?”) in Stigall’s and Zhang’s studies for not evaluating certain parameters beyond reporting no
significant differences among groups. *While the risk for reporting bias was interpreted as low (“+”) for quantitative aspects of Smith’s study,
having five open qualitative comments from their survey in their results did hamper interpreting the risk of bias. Therefore, settling on an unclear
(“?”) risk of bias was considered most appropriate.
Zhang’s study was interpreted as containing a high risk (“–”) for other biases. Firstly, the methods are unreproducible due to their use of a
3D–modeled non–market apparatus as one of the interventions, with no clear or reported specifications of the device. Secondly, intermittent
hemostasis was the co–intervention, but it was neither minimized nor standardized in the study’s design. Instead, only the animal subjects having
the 3D–modeled device as their hemorrhage intervention received intermittent hemostasis, making the study’s setting favorable for said subjects.
CS=Case Series; POS=Prospective observational study; RCAT=Randomized controlled animal trial.
126 | JSOM Volume 25, Edition 3 / Fall 2025