Page 120 - JSOM Fall 2018
P. 120
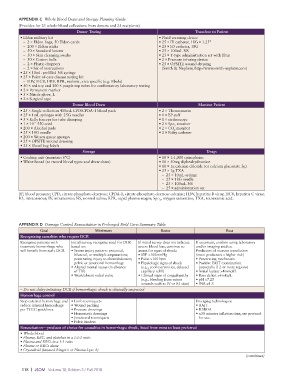
APPENDIX C Whole Blood Draw and Storage Planning Guide
(Provides for 25 wholeblood collections from donors and 25 recipients)
Donor Testing Transfuse to Patient
• Eldon military kit • Fluid warming device
– 2 Eldon bags, 50 Eldon cards • 25 IV catheter, 18G 1.25"
– 200 Eldon sticks • 25 IO catheter, 18G
– 50 Standard lancets • 25 100mL NS
– 50 Skin cleansing swabs • 25 Ytype administration set with filter
– 50 Cotton balls • 2 Pressure infusing device
– 2 Plastic droppers • 25 OPSITE wound dressing
– 2 Set of instructions (Smith & Nephew, http://www.smithnephew.com)
• 25 10mL prefilled NS syringe
• 25 Pointofcare disease testing kit
– HIV, HCV, HBV, RPR, malaria, area specific (e.g. Ebola)
• 50 red top and 100 purple top tubes for confirmatory laboratory testing
• 2 Permanent marker
• 5 Nitrile glove, L
• 2 Surgical tape
Donor Blood Draw Monitor Patient
• 25 Single collection 450mL CPD/CPDA1 blood pack • 2 Thermometer
• 25 1mL syringes with 25G needles • 1 BP cuff
• 5 Kelly forceps for tube clamping • 1 stethoscope
• 1 10" 550 cord • 2 Spo monitor
2
• 200 Alcohol pads • 2 CO monitor
2
• 25 18G needle • 2 Foley catheter
• 200 Woven gauze sponges
• 25 OPSITE wound dressing
• 25 Blood bag labels
Storage Drugs
• Cooling unit (maintain 6°C) • 10 1:1,000 epinephrine
• White board (to record blood types and draw dates) • 10 50mg diphenhydramine
• 10 1g calcium chloride (or calcium gluconate 3g)
• 25 1g TXA
– 25 10mL syringe
– 25 18G needle
– 25 100mL NS
– 25 administration set
BP, blood pressure; CPD, citratephosphatedextrose; CPDA1, citratephosphatedextroseadenine; HBV, hepatitis B virus; HCV, hepatitis C virus;
IO, intraosseous; IV, intravenous; NS, normal saline; RPR, rapid plasma reagin; Spo , oxygen saturation; TXA, tranexamic acid.
2
APPENDIX D Damage Control Resuscitation in Prolonged Field Care: Summary Table
Goal Minimum Better Best
Recognizing casualties who require DCR
Recognize patients with Initial survey, recognize need for DCR If initial survey does not indicate If uncertain, confirm using laboratory
traumatic hemorrhage who based on: severe blood loss, continue to and/or imaging studies.
will benefit from early DCR. • Severe injury pattern: proximal, assess for signs of shock: Predictors of massive transfusion
bilateral, or multiple amputations; • SBP <100mmHg (more predictors = higher risk)
penetrating injury to chest/abdomen; • Pulse >100 bpm • Penetrating mechanism
pelvic or junctional hemorrhage • Physiologic signs of shock • Positive FAST examination
• Altered mental status (in absence (e.g., cool extremities, delayed (especially if 2 or more regions)
of TBI) capillary refill) • Initial lactate >4mmol/L
• Weak/absent radial pulse • Clinical signs of coagulopathy • Base deficit >6mEq/L
(e.g., bleeding from minor • pH <7.25
wounds such as IV or IO sites) • INR ≥1.5
– Do not delay initiating DCR if hemorrhagic shock is clinically suspected
Hemorrhage control
Stop external hemorrhage and • Limb tourniquets Emerging technologies:
reduce internal hemorrhage • Wound packing • AAJT
per TCCC guidelines. • Pressure dressings • REBOA
• Hemostatic dressings • ≤30 minutes inflation time, see protocol
• Junctional tourniquets for use.
• Pelvic binders
Resuscitation—products of choice for casualties in hemorrhagic shock, listed from most to least preferred
• Whole blood
• Plasma, RBC, and platelets in a 1:1:1 ratio
• Plasma and RBCs in a 1:1 ratio
• Plasma or RBCs alone
• Crystalloid (lactated Ringer’s or Plasma-Lyte A)
(continues)
118 | JSOM Volume 18, Edition 3 / Fall 2018