Page 118 - JSOM Fall 2018
P. 118
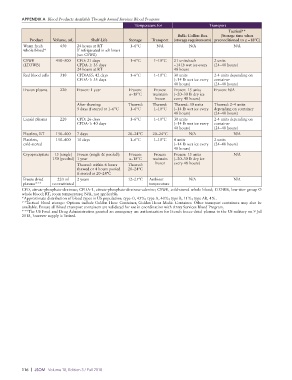
APPENDIX A Blood Products Available Through Armed Services Blood Program
Temperature for Transport
Tactical**
Bulk: Collins Box (Storage time when
Product Volume, mL Shelf-Life Storage Transport (storage requirements) preconditioned to ≤ −18°C)
Warm fresh 450 24 hours at RT 1–6°C N/A N/A N/A
whole blood* If refrigerated in ≤8 hours
(see CSWB)
CSWB 450–500 CPD: 21 days 1–6°C 1–10°C 21 units/each 2 units
(LTOWB) CPDA1: 35 days ~14 lb wet ice every (24–48 hours)
24 hours at RT 48 hours
Red blood cells 310 CPD/AS5: 42 days 1–6°C 1–10°C 30 units 24 units depending on
CPDA1: 35 days (~14 lb wet ice every container
48 hours) (24–48 hours)
Frozen plasma 220 Frozen: 1 year Frozen: Frozen: Frozen: 15 units Frozen: N/A
≤–18°C maintain (~20–30 lb dry ice
frozen every 48 hours)
After thawing: Thawed: Thawed: Thawed: 30 units Thawed: 2–4 units
5 days if stored at 1–6°C 1–6°C 1–10°C (~14 lb wet ice every depending on container
48 hours) (24–48 hours)
Liquid plasma 220 CPD: 26 days 1–6°C 1–10°C 30 units 24 units depending on
CPDA1: 40 days (~14 lb wet ice every container
48 hours) (24–48 hours)
Platelets, RT 150–400 7 days 20–24°C 20–24°C N/A N/A
Platelets, 150–400 10 days 1–6°C 1–10°C 4 units 2 units
coldstored (~14 lb wet ice every (24–48 hours)
48 hours)
Cryoprecipitate 15 (single) Frozen (single & pooled): Frozen: Frozen: Frozen: 15 units N/A
150 (pooled) 1 year ≤–18°C maintain (~20–30 lb dry ice
Thawed: within 6 hours Thawed: frozen every 48 hours)
thawed or 4 hours pooled 20–24°C
if stored at 20–24°C
Freeze dried 220 ml 2 years 12–25°C Ambient N/A N/A
plasma*** reconstituted temperature
CPD, citratephosphatedextrose; CPDA1, citratephosphatedextroseadenine; CSWB, coldstored whole blood; LTOWB, lowtiter group O
whole blood; RT, room temperature; N/A, not applicable.
*Approximate distribution of blood types in US population: type O, 45%; type A, 40%; type B, 11%; type AB, 4%.
**Tactical blood storage: Options include Golden Hour Container, Golden Hour Medic Container. Other transport containers may also be
available. Ensure all blood transport containers are validated for use in coordination with Army Services Blood Program.
***The US Food and Drug Administration granted an emergency use authorization for French freezedried plasma to the US military on 9 Jul
2018, however supply is limited.
116 | JSOM Volume 18, Edition 3 / Fall 2018