Page 69 - JSOM Fall 2023
P. 69
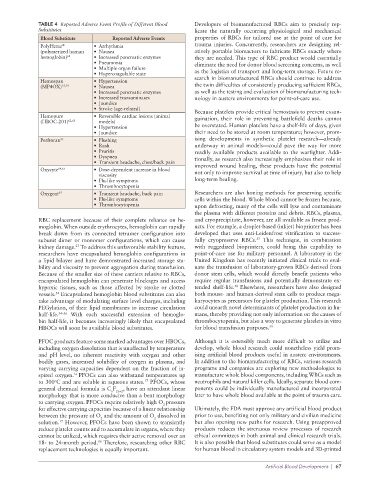
TABLE 4 Reported Adverse Event Profile of Different Blood Developers of biomanufactured RBCs aim to precisely rep-
Substitutes licate the naturally occurring physiological and mechanical
Blood Substitute Reported Adverse Events properties of RBCs for tailored use at the point of care for
PolyHeme • Arrhythmia trauma injuries. Concurrently, researchers are designing rel-
®
(polymerized human • Nausea atively portable bioreactors to fabricate RBCs exactly where
hemoglobin) 61 • Increased pancreatic enzymes they are needed. This type of RBC product would essentially
• Pneumonia eliminate the need for donor blood screening concerns, as well
• Multiple organ failure
• Hypercoagulable state as the logistics of transport and long-term storage. Future re-
Hemospan • Hypertension search in biomanufactured RBCs should continue to address
(MP4OX) 15,19 • Nausea the twin difficulties of consistently producing sufficient RBCs,
• Increased pancreatic enzymes as well as the testing and evaluation of biomanufacturing tech-
• Increased transaminases nology in austere environments for point-of-care use.
• Jaundice
• Stroke (age-related) Because platelets provide critical hemostasis to prevent exsan-
Hemopure • Reversible cardiac lesions (animal guination, their role in preventing battlefield deaths cannot
(HBOC-201) 62,63 models)
• Hypertension be overstated. Human platelets have a shelf-life of days, given
• Jaundice their need to be stored at room temperature; however, prom-
Perftoran 35 • Flushing ising developments in synthetic platelet research—already
• Rash underway in animal models—could pave the way for more
• Pruritis readily available products available to the warfighter. Addi-
• Dyspnea tionally, as research also increasingly emphasizes their role in
• Transient headache, chest/back pain improved wound healing, these products have the potential
Oxycyte 64,65 • Dose-dependent increase in blood not only to improve survival at time of injury, but also to help
viscosity
• Flu-like symptoms long-term healing.
• Thrombocytopenia
Oxygent 65 • Transient headache, back pain Researchers are also honing methods for preserving specific
• Flu-like symptoms cells within the blood. Whole blood cannot be frozen because,
• Thrombocytopenia upon defrosting, many of the cells will lyse and contaminate
the plasma with different proteins and debris. RBCs, plasma,
RBC replacement because of their complete reliance on he- and cryoprecipitate, however, are all available as frozen prod-
moglobin. When outside erythrocytes, hemoglobin can rapidly ucts. For example, a droplet-based (inkjet) bioprinter has been
break down from its connected tetramer configuration into developed that uses anti-Leidenfrost vitrification to success-
57
subunit dimer or monomer configurations, which can cause fully cryopreserve RBCs. This technique, in combination
kidney damage. To address this unfavorable stability feature, with ruggedized bioprinters, could bring this capability to
27
researchers have encapsulated hemoglobin configurations in point-of-care use for military personnel. A laboratory in the
a lipid bilayer and have demonstrated increased storage sta- United Kingdom has recently initiated clinical trials to eval-
bility and viscosity to prevent aggregation during transfusion. uate the transfusion of laboratory-grown RBCs derived from
Because of the smaller size of these carriers relative to RBCs, donor stem cells, which would directly benefit patients who
encapsulated hemoglobin can penetrate blockages and access require regular transfusions and potentially demonstrate ex-
hypoxic tissues, such as those affected by stroke or clotted tended shelf-life. Elsewhere, researchers have also designed
58
vessels. Encapsulated hemoglobin blood substitutes can also both mouse- and human-derived stem cells to produce mega-
54
take advantage of modulating surface level charges, including karyocytes as precursors for platelet production. This research
PEGylation, of their lipid membranes to increase circulation could unearth novel determinants of platelet production in hu-
half-life. 54–56 With each successful extension of hemoglo- mans, thereby providing not only information on the causes of
bin half-life, it becomes increasingly likely that encapsulated thrombocytopenia, but also a way to generate platelets in vitro
HBOCs will soon be available blood substitutes. for blood transfusion purposes. 59
PFOC products feature some marked advantages over HBOCs, Although it is ostensibly much more difficult to utilize and
including oxygen dissolution that is unaffected by temperature develop, whole blood research could nonetheless yield prom-
and pH level, no inherent reactivity with oxygen and other ising artificial blood products useful in austere environments.
bodily gases, increased solubility of oxygen in plasma, and In addition to the biomanufacturing of RBCs, various research
varying carrying capacities dependent on the fraction of in- programs and companies are exploring new methodologies to
spired oxygen. PFOCs can also withstand temperatures up manufacture whole blood components, including WBCs such as
16
to 300 C and are soluble in aqueous states. PFOCs, whose neutrophils and natural killer cells. Ideally, separate blood com-
17
o
general chemical formula is C F , have an attendant linear ponents could be individually manufactured and incorporated
n 2n+2
morphology that is more conducive than a bent morphology later to have whole blood available at the point of trauma care.
to carrying oxygen. PFOCs require relatively high O pressure
2
for effective carrying capacities because of a linear relationship Ultimately, the FDA must approve any artificial blood product
between the pressure of O and the amount of O dissolved in prior to use, benefiting not only military and civilian medicine
2
2
solution. However, PFOCs have been shown to transiently but also opening new paths for research. Using preapproved
17
reduce platelet counts and to accumulate in organs, where they products reduces the strenuous review processes of research
cannot be utilized, which requires their active removal over an ethical committees in both animal and clinical research trials.
18- to 24-month period. Therefore, researching other RBC It is also possible that blood substitutes could serve as a model
16
replacement technologies is equally important. for human blood in circulatory system models and 3D-printed
Artificial Blood Development | 67