Page 85 - JSOM Summer 2024
P. 85
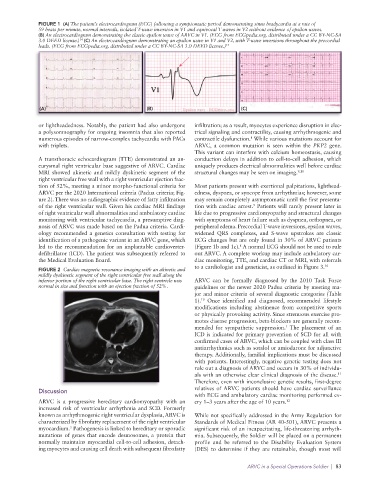
FIGURE 1 (A) The patient’s electrocardiogram (ECG) following a symptomatic period demonstrating sinus bradycardia at a rate of
59 beats per minute, normal intervals, isolated T-wave inversion in V1 and equivocal T waves in V2 without evidence of epsilon waves.
(B) An electrocardiogram demonstrating the classic epsilon wave of ARVC in V1. (ECG from ECGpedia.org. distributed under a CC BY-NC-SA
3.0 DEED license.) (C) An electrocardiogram demonstrating an epsilon wave in V1 and V2, with T-wave inversions throughout the precordial
14
leads. (ECG from ECGpedia.org. distributed under a CC BY-NC-SA 3.0 DEED license.) 14
(A) (B) (C)
or lightheadedness. Notably, the patient had also undergone infiltration; as a result, myocytes experience disruption in elec-
a polysomnography for ongoing insomnia that also reported trical signaling and contractility, causing arrhythmogenic and
numerous episodes of narrow-complex tachycardia with PACs contractile dysfunction. While various mutations account for
8
with triplets. ARVC, a common mutation is seen within the PKP2 gene.
This variant can interfere with calcium homeostasis, causing
A transthoracic echocardiogram (TTE) demonstrated an an- conduction delays in addition to cell-to-cell adhesion, which
eurysmal right ventricular base suggestive of ARVC. Cardiac uniquely produces electrical abnormalities well before cardiac
MRI showed akinetic and mildly dyskinetic segment of the structural changes may be seen on imaging. 9,10
right ventricular free wall with a right ventricular ejection frac-
tion of 52%, meeting a minor morpho-functional criteria for Most patients present with exertional palpitations, lighthead-
ARVC per the 2020 International criteria (Padua criteria; Fig- edness, dyspnea, or syncope from arrhythmias; however, some
ure 2). There was no radiographic evidence of fatty infiltration may remain completely asymptomatic until the first presenta-
of the right ventricular wall. Given his cardiac MRI findings tion with cardiac arrest. Patients will rarely present later in
9
of right ventricular wall abnormalities and ambulatory cardiac life due to progressive cardiomyopathy and structural changes
monitoring with ventricular tachycardia, a presumptive diag- with symptoms of heart failure such as dyspnea, orthopnea, or
nosis of ARVC was made based on the Padua criteria. Cardi- peripheral edema. Precordial T-wave inversions, epsilon waves,
ology recommended a genetics consultation with testing for widened QRS complexes, and S-wave upstrokes are classic
identification of a pathogenic variant in an ARVC gene, which ECG changes but are only found in 30% of ARVC patients
8
led to the recommendation for an implantable cardioverter- (Figure 1b and 1c). A normal ECG should not be used to rule
defibrillator (ICD). The patient was subsequently referred to out ARVC. A complete workup may include ambulatory car-
the Medical Evaluation Board. diac monitoring, TTE, and cardiac CT or MRI, with referrals
to a cardiologist and geneticist, as outlined in Figure 3. 10
FIGURE 2 Cardiac magnetic resonance imaging with an akinetic and
mildly dyskinetic segment of the right ventricular free wall along the
inferior portion of the right ventricular base. The right ventricle was ARVC can be formally diagnosed by the 2010 Task Force
normal in size and function with an ejection fraction of 52%. guidelines or the newer 2020 Padua criteria by meeting ma-
jor and minor criteria of several diagnostic categories (Table
10
1). Once identified and diagnosed, recommended lifestyle
modifications including abstinence from competitive sports
or physically provoking activity. Since strenuous exercise pro-
motes disease progression, beta-blockers are generally recom-
1
mended for sympathetic suppression. The placement of an
ICD is indicated for primary prevention of SCD for all with
confirmed cases of ARVC, which can be coupled with class III
antiarrhythmics such as sotalol or amiodarone for adjunctive
therapy. Additionally, familial implications must be discussed
with patients. Interestingly, negative genetic testing does not
rule out a diagnosis of ARVC and occurs in 30% of individu-
11
als with an otherwise clear clinical diagnosis of the disease.
Therefore, even with inconclusive genetic results, first-degree
Discussion relatives of ARVC patients should have cardiac surveillance
with ECG and ambulatory cardiac monitoring performed ev-
ARVC is a progressive hereditary cardiomyopathy with an ery 1–3 years after the age of 10 years. 12
increased risk of ventricular arrhythmia and SCD. Formerly
known as arrhythmogenic right ventricular dysplasia, ARVC is While not specifically addressed in the Army Regulation for
characterized by fibrofatty replacement of the right ventricular Standards of Medical Fitness (AR 40-501), ARVC presents a
myocardium. Pathogenesis is linked to hereditary or sporadic significant risk of an incapacitating, life-threatening arrhyth-
5
mutations of genes that encode desmosomes, a protein that mia. Subsequently, the Soldier will be placed on a permanent
normally maintains myocardial cell-to-cell adhesion, detach- profile and be referred to the Disability Evaluation System
ing myocytes and causing cell death with subsequent fibrofatty (DES) to determine if they are retainable, though most will
ARVC in a Special Operations Soldier | 83