Page 80 - JSOM Spring 2024
P. 80
3.5"×10", Model 8542028665, PlastCare USA, Chatsworth, Outcome measures were pooled, and standard deviations and
CA, USA; https://plastcareusa.com/). Group 2 consisted of two ranges were calculated and reported for comparison. Micro-
groups of five screws, each packaged with blue sterilization soft Excel (version 2019) was used to collect and analyze out-
cellulose wrap (Sterilization Wrap 30" × 30", Blue Autoclave come measures.
Film, Single Layer Cellulose, B091BBFHDL, AMZ Medical,
Libertyville, IL, USA; https://www.amzsupply.com/). Group 3 Results
consisted of two groups of five screws, each packaged in ster-
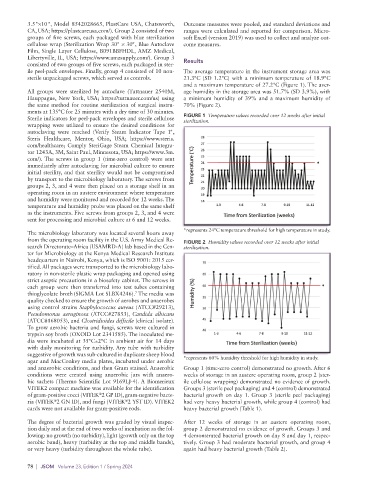
ile peel-pack envelopes. Finally, group 4 consisted of 10 non- The average temperature in the instrument storage area was
sterile unpackaged screws, which served as controls. 21.3°C (SD 1.2°C) with a minimum temperature of 18.9°C
and a maximum temperature of 27.2°C (Figure 1). The aver-
All groups were sterilized by autoclave (Tuttnauer 2540M, age humidity in the storage area was 51.7% (SD 3.9%), with
Hauppague, New York, USA; https://tuttnauer.com/us) using a minimum humidity of 39% and a maximum humidity of
the same method for routine sterilization of surgical instru- 70% (Figure 2).
ments at 135°C for 25 minutes with a dry time of 30 minutes.
Sterile indicators for peel-pack envelopes and sterile cellulose FIGURE 1 Temperature values recorded over 12 weeks after initial
sterilization.
wrapping were utilized to ensure the desired conditions for
autoclaving were reached (Verify Steam Indicator Tape 1",
Steris Healthcare, Mentor, Ohio, USA; https://www.steris.
com/healthcare; Comply SteriGage Steam Chemical Integra-
tor 1243A, 3M, Saint Paul, Minnesota, USA; https://www.3m.
com/). The screws in group 1 (time-zero control) were sent
immediately after autoclaving for microbial culture to ensure
initial sterility, and that sterility would not be compromised
by transport to the microbiology laboratory. The screws from
groups 2, 3, and 4 were then placed on a storage shelf in an
operating room in an austere environment where temperature
and humidity were monitored and recorded for 12 weeks. The
temperature and humidity probe was placed on the same shelf
as the instruments. Five screws from groups 2, 3, and 4 were
sent for processing and microbial culture at 6 and 12 weeks.
*represents 24°C temperature threshold for high temperature in study.
The microbiology laboratory was located several hours away
from the operating room facility in the U.S. Army Medical Re- FIGURE 2 Humidity values recorded over 12 weeks after initial
search Directorate–Africa (USAMRD-A) lab based in the Cen- sterilization.
ter for Microbiology at the Kenya Medical Research Institute
headquarters in Nairobi, Kenya, which is ISO 9001: 2015 cer-
tified. All packages were transported to the microbiology labo-
ratory in non-sterile plastic wrap packaging and opened using
strict aseptic precautions in a biosafety cabinet. The screws in
each group were then transferred into test tubes containing
thioglycolate broth (SIGMA Lot SLBX4246). The media was
7
quality checked to ensure the growth of aerobes and anaerobes
using control strains Staphylococcus aureus (ATCC#29213),
Pseudomonas aeruginosa (ATCC#27853), Candida albicans
(ATCC#168053), and Clostridioides difficile (clinical isolate).
To grow aerobic bacteria and fungi, screws were cultured in
trypsin soy broth (OXOID Lot 2341585). The inoculated me-
dia were incubated at 35°C±2°C in ambient air for 14 days
with daily monitoring for turbidity. Any tube with turbidity
suggestive of growth was sub-cultured in duplicate sheep blood
agar and MacConkey media plates, incubated under aerobic *represents 60% humidity threshold for high humidity in study.
and anaerobic conditions, and then Gram stained. Anaerobic Group 1 (time-zero control) demonstrated no growth. After 6
conditions were created using anaerobic jars with anaero- weeks of storage in an austere operating room, group 2 (ster-
bic sachets (Thermo Scientific Lot 9169LJ-4). A Biomerieux ile cellulose wrapping) demonstrated no evidence of growth.
VITEK2 compact machine was available for the identification Groups 3 (sterile peel packaging) and 4 (control) demonstrated
®
of gram-positive cocci (VITEK 2 GP ID), gram-negative bacte- bacterial growth on day 1. Group 3 (sterile peel packaging)
®
®
ria (VITEK 2 GN ID), and fungi (VITEK 2 YST ID). VITEK2 had very heavy bacterial growth, while group 4 (control) had
cards were not available for gram-positive rods. heavy bacterial growth (Table 1).
The degree of bacterial growth was graded by visual inspec- After 12 weeks of storage in an austere operating room,
tion daily and at the end of two weeks of incubation as the fol- group 2 demonstrated no evidence of growth. Groups 3 and
lowing: no growth (no turbidity), light (growth only on the top 4 demonstrated bacterial growth on day 8 and day 1, respec-
aerobic band), heavy (turbidity at the top and middle bands), tively. Group 3 had moderate bacterial growth, and group 4
or very heavy (turbidity throughout the whole tube). again had heavy bacterial growth (Table 2).
78 | JSOM Volume 23, Edition 1 / Spring 2024