Page 74 - JSOM Spring 2024
P. 74
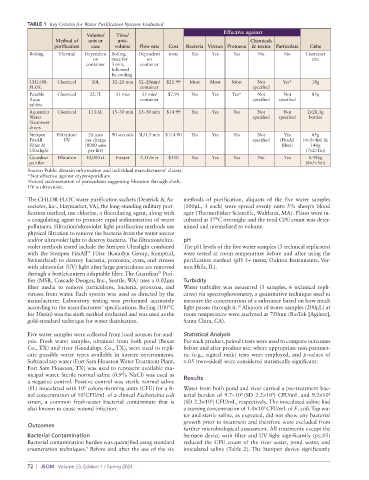
TABLE 1 Key Criteria for Water Purification Systems Evaluated
Effective against
Volume/ Time/
Method of unit or unit- Chemicals
purification case volume Flow rate Cost Bacteria Viruses Protozoa & toxins Particulate Cube
Boiling Thermal Dependent Boiling Dependent none Yes Yes Yes No No Container
on once for on size
container 3 min, container
followed
by cooling
CHLOR- Chemical 10L 12–20 min 12–20min/ $21.99 Most Most Most Not Yes* 18g
FLOC container specified
Potable Chemical 22.7L 35 min 35 min/ $7.95 Yes Yes Yes* Not Not 85g
Aqua container specified specified
tablets
Aquamira Chemical 113.6L 15–30 min 15–30 min $14.99 Yes Yes Yes Not Not 2×28.3g
Water specified specified bottles
Treatment
drops
Steripen Filtration/ 20 uses 90 seconds 3L/1.5 min $114.90 Yes Yes Yes Not Yes 65g
FitsAll UV per charge specified (FitsAll (4×3×4in) &
Filter & (8000 uses filter) 140g
Ultralight per life) (7×2×1in)
Guardian Filtration 10,000+L Instant 2.5L/min $350 Yes Yes Yes No Yes 0.49kg
purifier (8×5×3in)
Source: Public domain information and individual manufacturers’ claims.
*Not effective against cryptosporidium.
† Forced sedimentation of particulates suggesting filtration through cloth.
UV = ultraviolet.
The CHLOR-FLOC water purification sachets (Deatrick & As- methods of purification, aliquots of the five water samples
sociates, Inc., Haymarket, VA), the long-standing military puri- (100µL, 3 each) were spread evenly onto 5% sheep’s blood
fication method, use chlorine, a flocculating agent, along with agar (ThermoFisher Scientific, Waltham, MA). Plates were in-
a coagulating agent to promote rapid sedimentation of water cubated at 37°C overnight and the total CFU count was deter-
pollutants. Filtration/ultraviolet light purification methods use mined and normalized to volume.
physical filtration to remove the bacteria from the water source
and/or ultraviolet light to destroy bacteria. The filtration/ultra- pH
violet methods tested include the Steripen Ultralight combined The pH levels of the five water samples (3 technical replicates)
with the Steripen FitsAll Filter (Katadyn Group, Kempttal, were tested at room temperature before and after using the
™
Switzerland) to destroy bacteria, protozoa, cysts, and viruses purification method (pH 5+ meter, Oakton Instruments, Ver-
with ultraviolet (UV) light after large particulates are removed non Hills, IL).
through a bottle/canteen-adaptable filter. The Guardian Puri-
™
fier (MSR, Cascade Designs, Inc., Seattle, WA) uses a 0.02µm Turbidity
filter media to remove particulates, bacteria, protozoa, and Water turbidity was measured (3 samples, 6 technical repli-
viruses from water. Each system was used as directed by the cates) via spectrophotometry, a quantitative technique used to
manufacturer. Laboratory testing was performed accurately measure the concentration of a substance based on how much
according to the manufacturers’ specifications. Boiling (100°C light passes through it. Aliquots of water samples (200µL) at
10
for 30min) was the sixth method evaluated and was used as the room temperature were analyzed at 750nm (BioTek [Agilent],
gold-standard technique for water disinfection. Santa Clara, CA).
Five water samples were collected from local sources for anal- Statistical Analysis
ysis. Fresh water samples, obtained from both pond (Bexar For each product, paired t tests were used to compare outcomes
Co., TX) and river (Guadalupe Co., TX), were used to repli- before and after product use; where appropriate non-paramet-
cate possible water types available in austere environments. ric (e.g., signed rank) tests were employed, and p-values of
Softened tap water (Fort Sam Houston Water Treatment Plant, ≤.05 (two-sided) were considered statistically significant.
Fort Sam Houston, TX) was used to represent available mu-
nicipal water. Sterile normal saline (0.9% NaCl) was used as Results
a negative control. Positive control was sterile normal saline
8
(1L) inoculated with 10 colony-forming units (CFU) for a fi- Water from both pond and river carried a pre-treatment bac-
2
nal concentration of 10 CFU/mL of a clinical Escherichia coli terial burden of 9.7×10 (SD 2.2×10 ) CFU/mL and 9.2×10 2
5
2
strain, a common fresh-water bacterial contaminate that is (SD 2.3×10 ) CFU/mL, respectively. The inoculated saline had
2
also known to cause wound infection. a starting concentration of 1.0×10 CFU/mL of E. coli. Tap wa-
5
ter and sterile saline, as expected, did not show any bacterial
growth prior to treatment and therefore were excluded from
Outcomes
further microbiological assessment. All treatments except the
Bacterial Contamination Steripen device with filter and UV light significantly (p≤.05)
Bacterial contamination burden was quantified using standard reduced the CFU count of the river water, pond water, and
enumeration techniques. Before and after the use of the six inoculated saline (Table 2). The Steripen device significantly
9
72 | JSOM Volume 23, Edition 1 / Spring 2024