Page 32 - JSOM Fall 2020
P. 32
Q5. What are the required characteristics of a portable IV To date, there is no optimal portable fluid/blood-warming
warming device for infusion of fluids and blood products? device that has been evaluated independently among other de-
Resuscitation with blood products facilitates a return to vices with ideal features and performance characteristics for
baseline aerobic metabolism, increasing the body’s intrinsic use on the battlefield. New products are in development. See
ability to produce heat. Blood must be stored at 4°C–8°C Table 4 for recommended ideal device specifications and per-
(39°F–46°F); however, infusion of fluid at this temperature formance characteristics. 26
leads to a drop in core temperature. With as little as 500mL
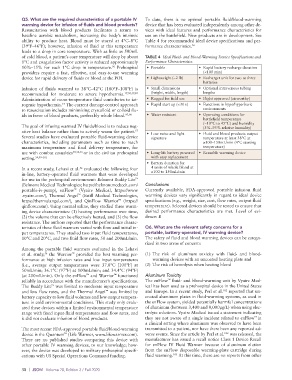
of cold blood, a patient’s core temperature will drop by about TABLE 4 Ideal Fluid- and Blood-Warming Device Specifications and
1°C and coagulation factor activity is reduced approximately Performance Characteristics
10%–15% for each 1°C drop in temperature. Prehospital • Portable • Rapid battery recharge duration
96
providers require a fast, effective, and easy-to-use warming (<100 min)
device for rapid delivery of fluids or blood at the POI. • Lightweight (~2 lb) • Recharger unit for two to three
batteries
Infusion of fluids warmed to 38°C–42°C (100°F–108°F) is • Small dimensions • Optional intravenous tubing
recommended for moderate to severe hypothermia. 17,50,57,82 (height, width, length) lengths
Administration of room-temperature fluid contributes to iat- • Rugged for field use • Flight approved (airworthy)
rogenic hypothermia. The current damage-control approach • Rapid start up (<30 s) • Functions in hypo/hyperbaric
13
to resuscitation includes minimizing crystalloid or colloid flu- environments
ids in favor of blood products, preferably whole blood. 97,98 • Water resistant • Operating conditions for
battlefield temperature
(−10°C to 45°C) and humidity
The goal of infusing warmed IV fluids/blood is to reduce neg- (5%–95% relative humidity)
ative heat balance rather than to actively warm the patient. • Low noise and light • Fluid and blood products output
57
Several studies have evaluated portable fluid-warming device signature temperature at least 38°C at
characteristics, including parameters such as time to reach ≥100–150mL/min (4°C starting
maximum temperature and final delivery temperature, for temperature)
use with combat casualties 50,54,56 or in the civilian prehospital • Long-life battery powered • Reusable warming device
setting. 59,99–101 with easy replacement
• Battery duration for
In a recent study, Lehavi et al. evaluated the following four 4 units of whole blood at
56
in-line, battery-operated fluid warmers that were developed ≥100 to 150mL/min
for use in the prehospital environment: Belmont Buddy Lite
™
(Belmont Medical Technologies; https://belmontmedtech.com/ Conclusions
portable-iv-pump), enFlow (Vyaire Medical, https://www Currently available, FDA-approved portable infusion fluid
™
.vyaire.com/), Thermal Angel (Estill Medical Technologies, warm ing devices vary significantly in regard to ideal device
™
https://thermalangel.com/), and QinFlow Warrior (https:// specifications (e.g., weight, size, cost, flow rates, output fluid
™
qinflow.com/). Using normal saline, they studied three warm- temperature). Selected devices should be tested to ensure that
ing device characteristics: (1) heating performance over time, desired performance characteristics are met. Level of evi-
(2) the volume that can be effectively heated, and (3) the flow dence: B
resistance. The authors reported that the performance charac-
teristics of these fluid warmers varied with flow and initial in- Q6. What are the relevant safety concerns for a
put temperatures. They studied two input fluid temperatures, portable, battery-operated, IV warming device?
10°C and 20°C, and two fluid flow rates, 50 and 200mL/min. The safety of fluid and blood warming devices can be catego-
rized in two areas of concern:
Among the portable fluid warmers evaluated in the Lehavi
et al. study, the Warrior provided the best warming per- (1) The risk of aluminum toxicity with fluid- and blood-
56
™
formance at high infusion rates and low input temperatures warming devices with an uncoated heating plate and
(i.e., average output temperatures were 37.8°C [100°F] at (2) The risk of hemolysis when heating blood
50mL/min; 36.1°C (97°F) at 100mL/min; and 34.4°C (94°F)
(at 200mL/min). Only the enFlow and Warrior functioned Aluminum Toxicity
™
™
reliably in accordance with the manufacturer’s specifications. The enFlow fluid- and blood-warming unit by Vyaire Med-
™
The Buddy Lite was limited to moderate input temperature ical has been used as a prehospital device in the United States
™
and low flow rates, and the Thermal Angel was limited by and Europe. In a recent study, Perl et al. reported that un-
102
™
battery capacity to low fluid volumes and low output tempera- coated aluminum plates in fluid-warming systems, as used in
ture in cold environmental conditions. This study only evalu- the enFlow system, yielded potentially harmful concentrations
ated these devices within a limited environmental temperature of aluminum (between 3,400 and 8,000µg/L) when using elec-
range with fixed input-fluid temperatures and flow rates, and trolyte solutions. Vyaire Medical issued a statement indicating
it did not evaluate infusion of blood products. they are not aware of a single incident related to enFlow in
™
a clinical setting where aluminum was observed to have been
The most recent FDA-approved portable fluid/blood- warming transmitted to a patient, nor have there been any reported ad-
102
device is the Quantum (Life Warmer, www.lifewarmer.com). verse events. Since the article by Perl et al. was released, the
™
There are no published studies comparing this device with manufacturer has issued a recall notice Class 1 Device Recall
other portable IV warming devices, to our knowledge; how- for enFlow IV Fluid Warmer because of aluminum elution
ever, the device was developed to military prehospital specifi- from the enFlow disposable warming-plate cartridge during
103
cations with US Special Operations Command funding. fluid warming. At this time, there are no reports from other
30 | JSOM Volume 20, Edition 3 / Fall 2020