Page 85 - JSOM Fall 2023
P. 85
FIGURE 2. (A) White phosphorus burn, (B) orange related to a
chemical dye.
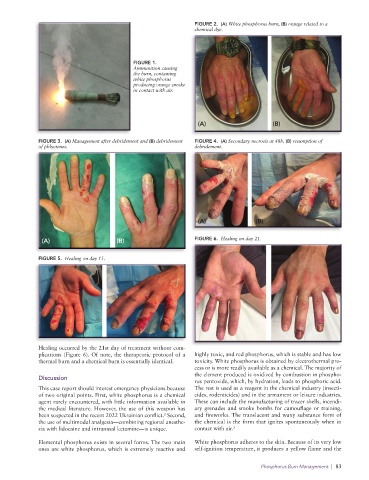
FIGURE 1.
Ammunition causing
the burn, containing
white phosphorus
producing orange smoke
in contact with air.
(A) (B)
Fig. 1 Ammunition causing the burn, containing white phosphorus producing orange smoke in contact with air
FIGURE 3. (A) Management after debridement and (B) debridement FIGURE 4. (A) Secondary necrosis at 48h, (B) resumption of
of phlyctenes. debridement.
(A) (B)
(A) (B) FIGURE 6. Healing on day 21.
FIGURE 5. Healing on day 15.
Healing occurred by the 21st day of treatment without com-
plications (Figure 6). Of note, the therapeutic protocol of a highly toxic, and red phosphorus, which is stable and has low
thermal burn and a chemical burn is essentially identical. toxicity. White phosphorus is obtained by electrothermal pro-
cess or is more readily available as a chemical. The majority of
the element produced is oxidized by combustion in phospho-
Discussion
rus pentoxide, which, by hydration, leads to phosphoric acid.
This case report should interest emergency physicians because The rest is used as a reagent in the chemical industry (insecti-
of two original points. First, white phosphorus is a chemical cides, rodenticides) and in the armament or leisure industries.
agent rarely encountered, with little information available in These can include the manufacturing of tracer shells, incendi-
the medical literature. However, the use of this weapon has ary grenades and smoke bombs for camouflage or training,
been suspected in the recent 2022 Ukrainian conflict. Second, and fireworks. The translucent and waxy substance form of
2
the use of multimodal analgesia––combining regional anesthe- the chemical is the form that ignites spontaneously when in
sia with lidocaine and intranasal ketamine––is unique. contact with air. 3
Elemental phosphorus exists in several forms. The two main White phosphorus adheres to the skin. Because of its very low
ones are white phosphorus, which is extremely reactive and self-ignition temperature, it produces a yellow flame and the
Phosphorus Burn Management | 83