Page 53 - Journal of Special Operations Medicine - Fall 2015
P. 53
85mL: 33%; and 64mL: 17% versus control animals: To examine these potential safety risks, we conducted
8%; p < .05). The hemorrhage rate was also reduced a dose-finding safety study in a splenic-hemorrhage
20
in all groups relative to the control group (p < .05) be- survival model with swine undergoing splenic injury,
tween two- and 10-fold at the lowest and highest doses, foam treatment, explantation, and subsequent recovery
respectively. Finally, we observed a rapid, transient, and and survival for 28 days and 90 days . Treatment with
23
21
dose-dependent increase in intra-abdominal pressure as- foam and its subsequent explantation did not adversely
sociated with foam expansion, which was well tolerated impact long-term survival at doses of 120mL or less
by experimental animals. 20 (120mL: n = 6; 100mL: n = 12). All animals required
some level of enteric repair associated with bowel le-
We developed a second swine model to determine the ef- sions (100mL: 3 ± 2 lesions, 120mL: 6 ± 3 lesions). One
fectiveness of foam in a TCCC resuscitation-compliant animal at the 100mL dose developed postoperative ileus
model of severe arterial hemorrhage (high flow, high following repair and was euthanized for experimental
pressure). 19,27 Two foam doses (100mL [n = 12] and reasons, rather than attempting corrective surgery or
120mL [n = 13]) were tested in this model compared nasogastric decompression consistent with clinical prac-
with a control group (n = 14). Median survival times tice. This result is consistent with the reported com-
21
were 135 minutes and 175 minutes for the 120mL and plication rates of 10% to 20% observed after bowel
100mL doses, respectively, compared with 32 minutes in repairs in human patients. 28–33 Further, many patients
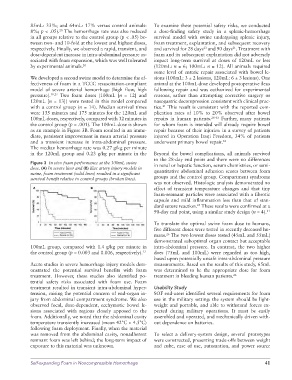
the control group (p < .001). The 100mL dose is shown for whom foam is intended will already require bowel
as an example in Figure 3B. Foam resulted in an imme- repair because of their injuries: in a survey of patients
diate, persistent improvement in mean arterial pressure injured in Operation Iraqi Freedom, 34% of patients
and a transient increase in intra-abdominal pressure. underwent primary bowel repair. 32
The median hemorrhage rate was 0.27 g/kg per minute
in the 120mL group and 0.23 g/kg per minute in the Beyond the bowel complications, all animals survived
to the 28-day end point and there were no differences
Figure 3 In vivo foam performance at the 100mL swine in renal or hepatic function, serum chemistries, or semi-
dose. (A) In severe liver and (B) iliac artery injury models in
swine, foam treatment (solid lines) resulted in a significant quantitative abdominal adhesion scores between foam
survival benefit relative to control groups (broken lines). groups and the control group. Compartment syndrome
was not observed. Histologic analysis demonstrated no
effect of transient temperature changes and that tiny
foam-remnant particles were associated with a fibrotic
capsule and mild inflammation less than that of stan-
dard suture reaction. These results were confirmed at a
23
90-day end point, using a similar study design (n = 4). 21
To translate the optimal swine foam dose to humans,
five different doses were tested in recently deceased hu-
mans. The two lowest doses tested (45mL and 55mL)
26
demonstrated suboptimal organ contact but acceptable
100mL group, compared with 1.4 g/kg per minute in intra-abdominal pressure. In contrast, the two higher
the control group (p = 0.003 and 0.006, respectively). 22 does (75mL and 100mL) were regarded as too high,
based upon potentially unsafe intra-abdominal pressure
Acute studies in severe hemorrhage injury models dem- measurements. Based on the results of this study, 65mL
onstrated the potential survival benefits with foam was determined to be the appropriate dose for foam
treatment. However, these studies also identified po- treatment in bleeding human patients. 26
tential safety risks associated with foam use. Foam
treatment resulted in transient intra-abdominal hyper- Usability Study
tension, raising the potential concern of end-organ in- SOF end-users identified several requirements for foam
jury from abdominal compartment syndrome. We also use in the military setting: the system should be light-
observed focal, dose-dependent, ecchymotic bowel le- weight and portable, and able to withstand forces ex-
sions associated with regions closely apposed to the pected during military operations. It must be easily
foam. Additionally, we noted that the abdominal cavity assembled and operated, and mechanically driven with-
temperature transiently increased (mean 42°C ± 4.5°C) out dependence on batteries.
following foam deployment. Finally, when the material
was removed from the abdominal cavity, nonadherent To select a delivery-system design, several prototypes
remnant foam was left behind; the long-term impact of were constructed, presenting trade-offs between weight
exposure to this material was unknown. and cube, ease of use, automation, and power source
Self-expanding Foam in Noncompressible Hemorrhage 41