Page 17 - JSOM Fall 2019
P. 17
central pulmonary embolus (PE); however, the report noted TABLE 1 Sample Differential Diagnosis for Life-Threatening Causes
suboptimal contrast bolus timing. Repeat laboratory results of Dyspnea
and imaging ordered earlier in the day by the patient’s pri- Cardiac Output/Vascular Respiratory Muscle
mary care physician were significant for D-dimer 0.54μg/mL • Ischemia Weakness
FEU, creatinine 1.27mg/dL, and anion gap 17mmol/L. Repeat • Arrhythmia • Guillain-Barré syndrome
• High-output heart failure
• Myasthenia gravis
CTPA was negative for central or segmental PE. • Tamponade • Multiple sclerosis
• Valve dysfunction • Botulism
Despite not taking any prescriptions or supplements at the • Pulmonary embolism • Chest wall trauma
time of our assessment, further history revealed that he had • Acute blood loss • Increased abdominal
completed a 14-day course of PQ (15mg base daily) 1 week pressure
earlier. Venous co-oximetry confirmed an elevated methemo- Lower Respiratory Tract Abnormality Upper Airway Obstruction
• Infection
• Inhaled foreign body
globin (MetHb) level of 8%. Military medical records showed • Potential bioterrorism agents (e.g., • Abscess
that the patient’s G6PD deficiency screening was negative. Af- anthrax, tularemia, Hantavirus) • Anaphylaxis
ter consultation with the Poison Control Center and discus- • Asthma Hemoglobin Abnormality
sion with the patient, we initiated treatment with methylene • Bronchitis • Anemia
blue intravenous infusion at a dose of 1mg/kg. Within minutes • Aspiration • Hemoglobinopathies
• Pneumothorax
of beginning treatment, we observed complete resolution of • Inhaled toxins, such as (e.g., thalassemia)
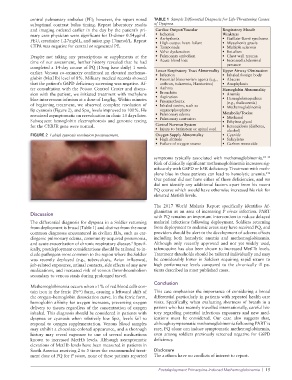
lip cyanosis (Figure 2), and Spo levels improved to 100%. He organophosphates • Methemoglobinemia
2
remained asymptomatic on reevaluation in clinic 13 days later. • Pulmonary edema Metabolic/Toxins
• Methanol
Subsequent hemoglobin electrophoresis and genomic testing • Pulmonary contusion • Ethylene glycol
for the CYB5R gene were normal. Central Nervous System • Ketoacidosis (diabetes,
• Injury to brainstem or spinal cord alcohol)
FIGURE 2 Labial cyanosis resolution posttreatment. Oxygen Supply Abnormality • Cyanide
• High altitude • Salicylates
• Failure of oxygen source • Carbon monoxide
symptoms typically associated with methemoglobinemia. 10–13
Risk of clinically significant methemoglobinemia increases sig-
nificantly with G6PD or b5R deficiency. Treatment with meth-
141
ylene blue in these patients can lead to hemolytic anemia.
Our patient did not have either of these deficiencies, and we
did not identify any additional factors apart from his recent
PQ course which would have otherwise increased his risk for
elevated MetHb levels.
The 2017 World Malaria Report specifically identifies Af-
ghanistan as an area of increasing P vivax infection. PART
Discussion
with PQ remains an important intervention to reduce delayed
The differential diagnosis for dyspnea in a Soldier returning malarial infections following deployment. Soldiers returning
from deployment is broad (Table 1) and distinct from the most from deployment to endemic areas may have received PQ, and
common diagnoses encountered in civilian EDs, such as car- providers should be alert to the development of adverse effects
diogenic pulmonary edema, community-acquired pneumonia, including both hemolytic anemia and methemoglobinemia.
and acute exacerbation of chronic respiratory disease. Specif- Although only recently approved and not yet widely used,
9
ically, postdeployment considerations should be tailored to in- tafeno quine has also been shown to increased MetHb levels.
clude pathogens more common to the region where the Soldier Treatment thresholds should be tailored individually and may
was recently deployed (e.g., tuberculosis, Avian influenza), be considerably lower in Soldiers requiring rapid return to
job-related exposures, animal contacts, side effects of any new high performance levels compared to the chronically ill pa-
medications, and increased risk of venous thromboembolism tients described in most published cases.
secondary to venous stasis during prolonged travel.
Conclusion
Methemoglobinemia occurs when >1% of red blood cells con-
tain iron in the ferric (Fe ) form, causing a leftward shift of This case emphasizes the importance of considering a broad
3+
the oxygen–hemoglobin dissociation curve. In the ferric form, differential particularly in patients with repeated health care
hemoglobin affinity for oxygen increases, preventing oxygen visits. Specifically, when evaluating shortness of breath in a
delivery to tissues regardless of the concentration of oxygen patient who has recently travelled internationally, careful his-
inhaled. This diagnosis should be considered in patients with tory regarding potential infectious exposures and new med-
dyspnea or cyanosis when relatively low Spo levels fail to ications must be considered. Our case also suggests that,
2
respond to oxygen supplementation. Venous blood samples although symptomatic methemoglobinemia following PART is
may exhibit a chocolate-colored appearance, and a thorough rare, PQ alone can induce symptomatic methemoglobinemia,
history may reveal exposure to one of several medications even among soldiers previously screened negative for G6PD
known to increased MetHb levels. Although asymptomatic deficiency.
elevations of MetHb levels have been measured in patients in
South America receiving 2 to 5 times the recommended treat- Disclosure
ment dose of PQ for P vivax, none of these patients reported The authors have no conflicts of interest to report.
Postdeployment Primaquine-Induced Methemoglobinemia | 15